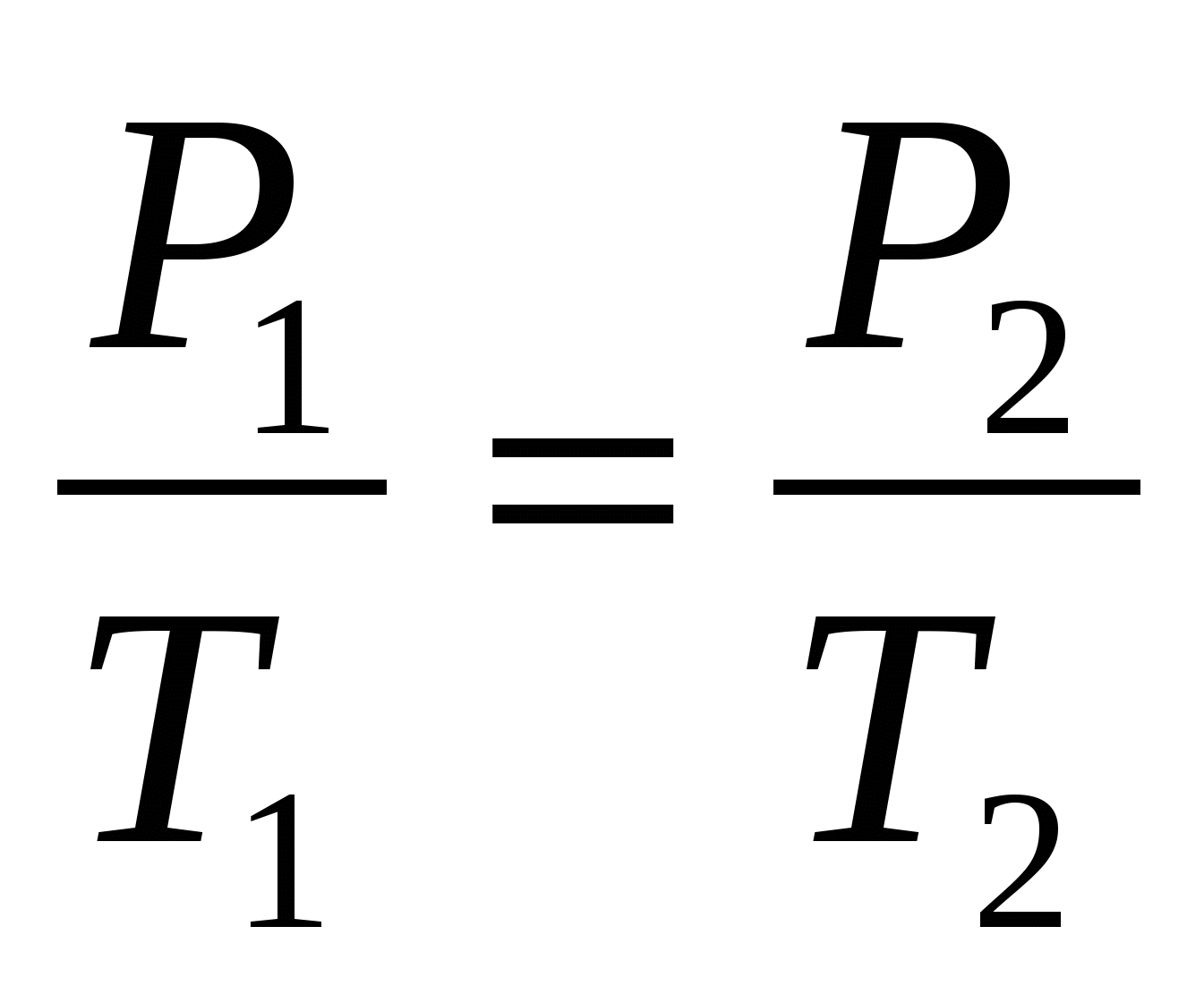Gay-Lussac’s law, Amontons’ law or the pressure law was found by Joseph Louis Gay-Lussac in 1809. It states that, for a given mass and constant volume of an ideal gas, the pressure exerted on the sides of its container is directly proportional to …
Discovered by Joseph Louis Gay-Lussac in the early 1800’s. That is pretty much all the ChemTeam knows. Maybe I’ll learn more of the details someday.
Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, change gravity, and more. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other.

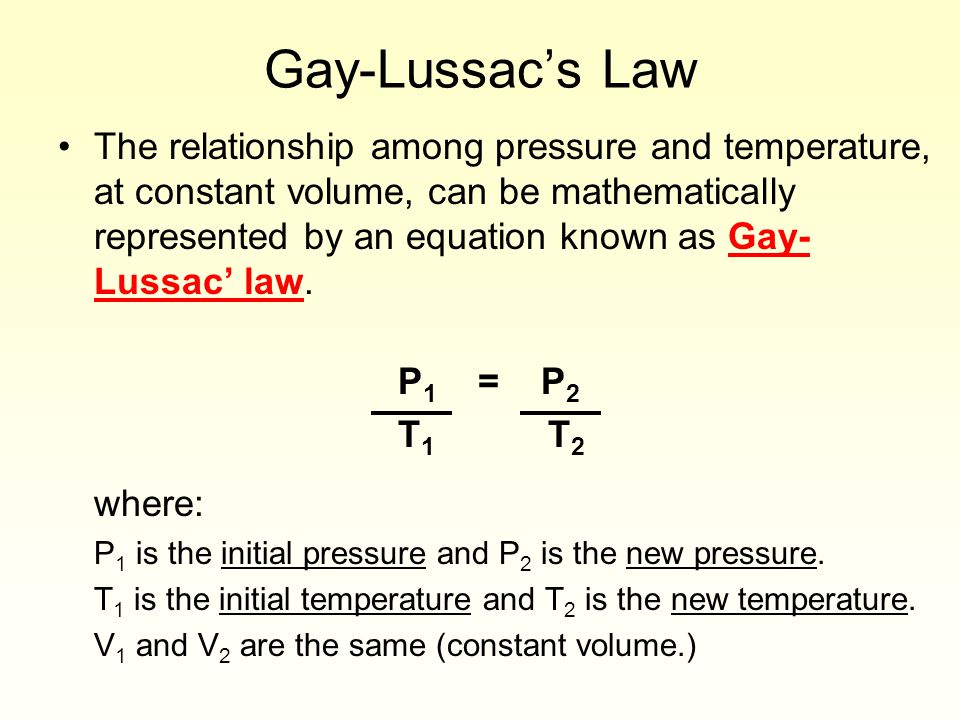
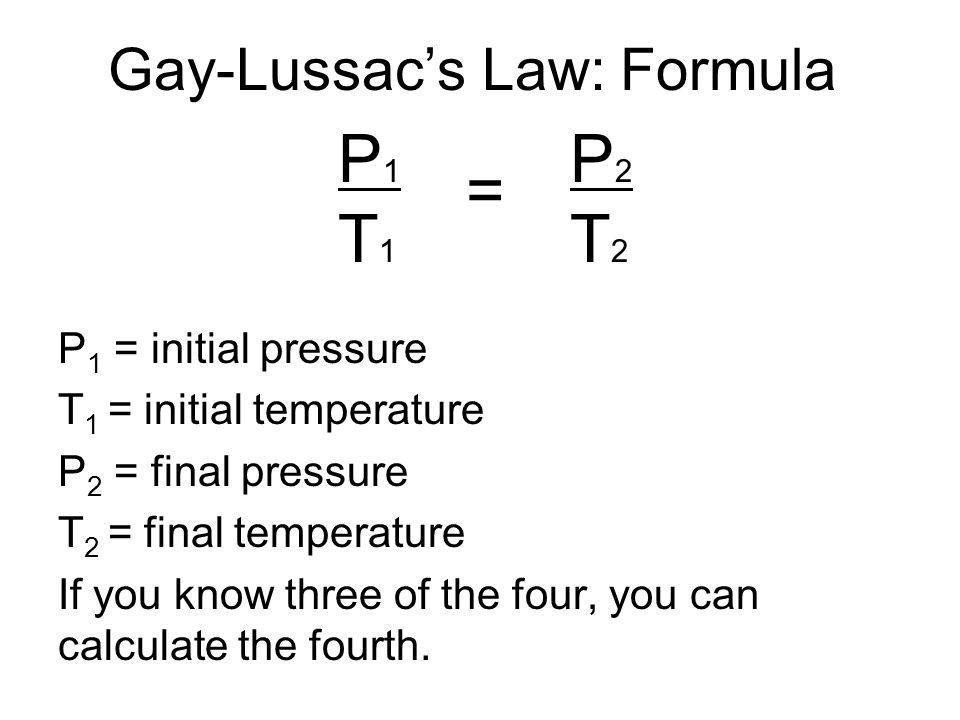
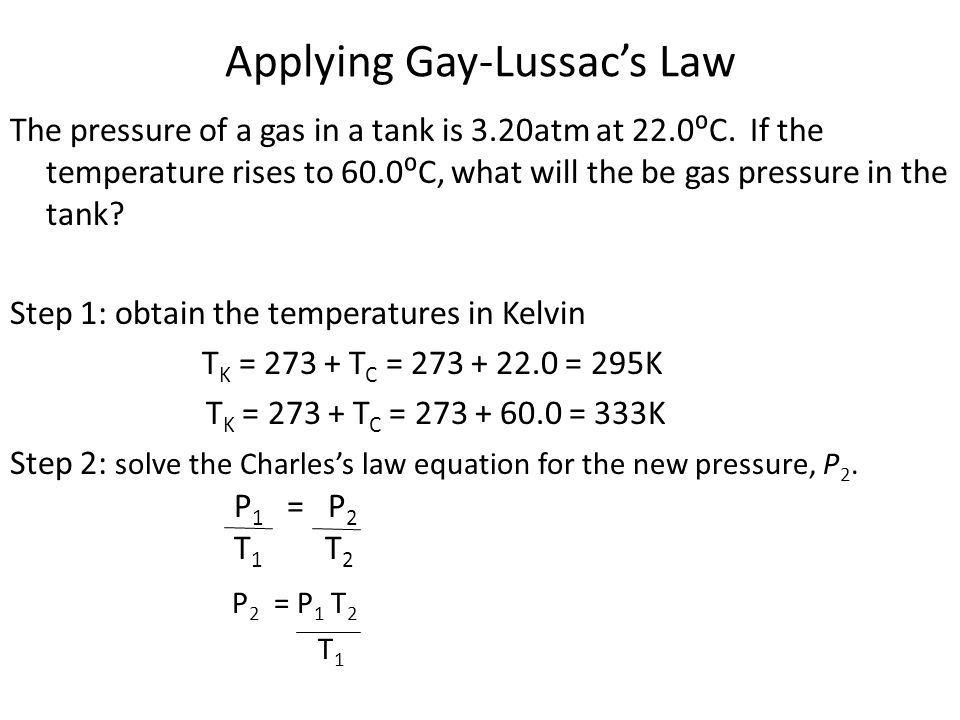
More advanced ideas involving gases gas law calculations involving manle’s Law, Charles’s Law, Gay-Lussac Law, P1V1/T1 = P2V2/T2, the ideal gas equation PV=nRT, ideal gas theory, how to determine the relative molecular mass Mr of a volatile liquid, Dalton’s Law of partial pressures, ideal gas behaviour and non-ideal gas behaviour, Graham’s Law





Gas Laws One of the most amazing things about gases is that, despite wide differences in chemical properties, all the gases more or less obey the gas laws.The gas laws deal with how gases behave with respect to pressure, volume, temperature, and amount.
When dealing with gases and the gas laws, you will need to solve for, or plug into an equation, values for many different quantities. Paying attention to units is important.
Gay-Lussac’s Law of Combining Gas Volumes tutorial with worked examples for chemistry students.
Gay Lussac’s Law Basic Concept Gay Lussac’s Law. Gay-Lussac’s Law states that the pressure of a sample of gas at constant volume, is …
Gay-Lussac’s law can refer to several discoveries made by French chemist Joseph Louis Gay-Lussac (1778–1850) and other scientists in the late 18th and early 19th centuries pertaining to thermal expansion of gases and the relationship between temperature, volume, and pressure.
You may know that you aren’t supposed to put an aerosol can in a fire because it could explode, but do you know why? In this lesson, we will explain Gay-Lussac’s law, which shows the relationship between the temperature and pressure of a gas.